Enzyme important for protein folding caught in the act
 Photo: Fotolia
Photo: Fotolia
A very important enzyme, the elongator, has been caught in the act. This complex is important for proper folding of all proteins present in cells of organisms as different as bacteria, animals, plants and fungi. Polish scientists took part in the research project.
Proteins are basic sets of building blocks from which the cell is built and which drive it to life. Each cell must constantly produce such proteins. This takes place in ribosomes. This is where mRNA brings the protein recipe extracted from DNA, and tRNA supplies appropriate amino acids - protein components.
But all this work must proceed at the right pace. After all, proteins have complex, three-dimensional structures. If the process of combining amino acids into proteins goes too fast or too slowly, proteins will fold incorrectly. And an incorrectly folded protein does not work as it should.
It has already been known that the rate of production of each protein is influenced by a protein complex - the elongator. It is responsible for the introduction of chemical modifications in tRNA. Previously, it has been shown that if the elongator function is abnormal, the dynamics of ribosomes fail - degenerated or incorrectly folded proteins and intracellular protein aggregates are formed.
In people, mutation in any of the six subunits of the elongator is associated with serious diseases. These include familial dysautonomia (a disease of the autonomic nervous system), obesity, bronchial asthma, intellectual disability, ventricular hypertrophy, Rolandic epilepsy and cancer.
Elongator is so important that it is present in the cells of bacteria, plants, fungi and animals. Researchers decided to understand how this complex works and capture it in action. The research results have been published in "Science Advances".
"Our paper describes how tRNA is bound in the active part of the complex" - says Dr. Sebastian Glatt, leader of the Max Planck research group at the Małopolska Centre of Biotechnology of the Jagiellonian University and one of the correspondence authors of the study.
Researchers also hope that in the future it will be possible to design drugs against diseases linked to the elongator.
"After ten years of research on this large cellular machine, we are finally beginning to have a clearer picture of the complex interactions between the various elongator subunits and the bound tRNA substrate that allow modification to occur" - say Dr Christoph W. Müller (EMBL in Heidelberg) and Dr. Jan Kosinski (EMBL in Hamburg).
Researchers used cryogenic electron microscopy - cryo-EM (the development of this method was awarded with the Nobel Prize in Chemistry in 2017). "In structural biology, it`s difficult to observe dynamic processes, but it is possible to capture something like time-lapse images. We want to capture structures showing various stages of tRNA modification, which will allow us to draw conclusions about the molecular mechanism of the modification reaction" - says Dr. Marcin Jaciuk from the Małopolska Centre of Biotechnology.
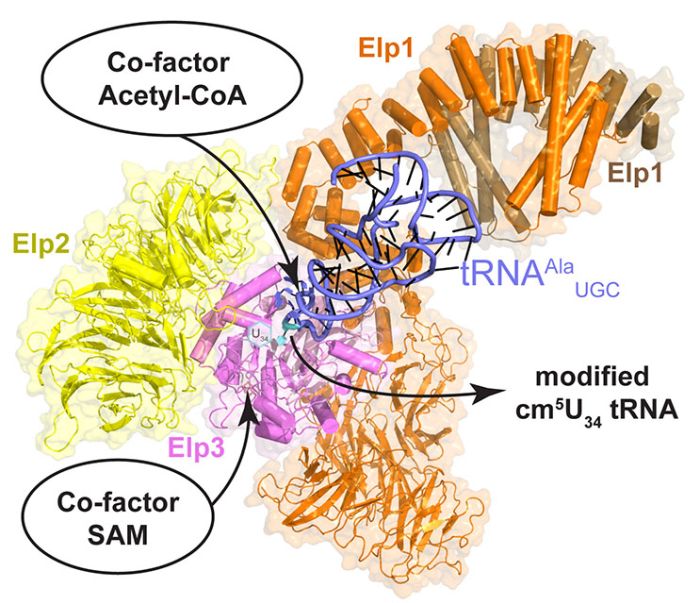
Structure of the elongator - an enzyme important for protein folding. Source: MCB UJ
According to the Małopolska Centre of Biotechnology representatives, top-class cryo-EM will soon be available in Poland. A microscope equipped with the latest, direct electron detectors has been installed in the SOLARIS National Synchrotron Radiation Centre of the Jagiellonian University. The microscope is currently being tested; after tests it will be made available to scientists. Information on the application for measurement time on the microscope can be found on the Solaris website.
Research in Kraków was financed with the National Science Centre OPUS grant and the Homing and Team Tech Core Facility programmes of the Foundation for Polish Science, with the support of Structural Biology Core Facility at the Małopolska Centre of Biotechnology.
PAP - Science in Poland, Ludwika Tomala
lt/ ekr/ kap/
tr. RL
Przed dodaniem komentarza prosimy o zapoznanie z Regulaminem forum serwisu Nauka w Polsce.